The Fractionation of Aliphatic and Aromatic Hydrocarbons from a Petroleum Extract Is Messy Business – Extractable Petroleum Hydrocarbons
Most states have their method for extraction and fractionation, or they borrow one from another state. The sample is extracted with methylene chloride, solvent exchanged into n-hexane, and concentrated to a final volume. Fractionation is performed on a small 3 to 5g. silica gel column that has been pre-rinsed with n-hexane. The aliphatic hydrocarbons are collected by adding hexane to the column and collecting. The aromatic hydrocarbons are collected by eluting the column with methylene chloride and collecting.
Have you ever noticed that your method works perfectly in winter but not in the summer? Silica is naturally hygroscopic and will absorb humidity from the room air. That’s why it makes a great desiccant. You fine-tuned your fractionation method in either a dry or humid season, and when the humidity changes, it doesn’t work! You are seeing either aromatics in your aliphatic fraction or aliphatics in your aromatic fraction.
How frustrating!
Here are a couple of solutions to this problem, but you will need permission from your state regulators before using them.
- Try pre-rinsing your silica gel columns with dry acetone. This will help your silica gel columns at a consistent level of activation.
- Use UCT’s Enviro-Clean FUSION Ag+ SPE column for your fractionation.
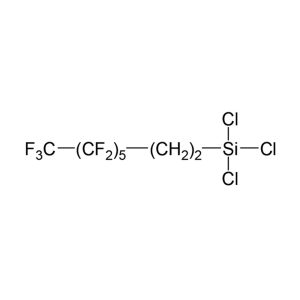
The new EPH fractionation sorbent consists of silver ions functionalized onto a solid
support. Aromatic hydrocarbons are selectively retained on the sorbent by forming a charge-transfer complex with the silver ions. This novel chemistry ensures high capacity for the aromatic hydrocarbons and no breakthrough into the aliphatic fraction. Consistent lot-to-lot reproducibility without optimizing the elution solvent for each batch of cartridges is the most significant advantage of this new sorbent. In addition, the fractionation protocol is easier, faster, and uses less solvent than silica cartridges. Also, using acetone instead of dichloromethane as the elution solvent for the aromatic fraction is more environmentally friendly.